Artificial blood, products using different proteins, new formulations and new uses for plasma may all affect our industry in the near future.
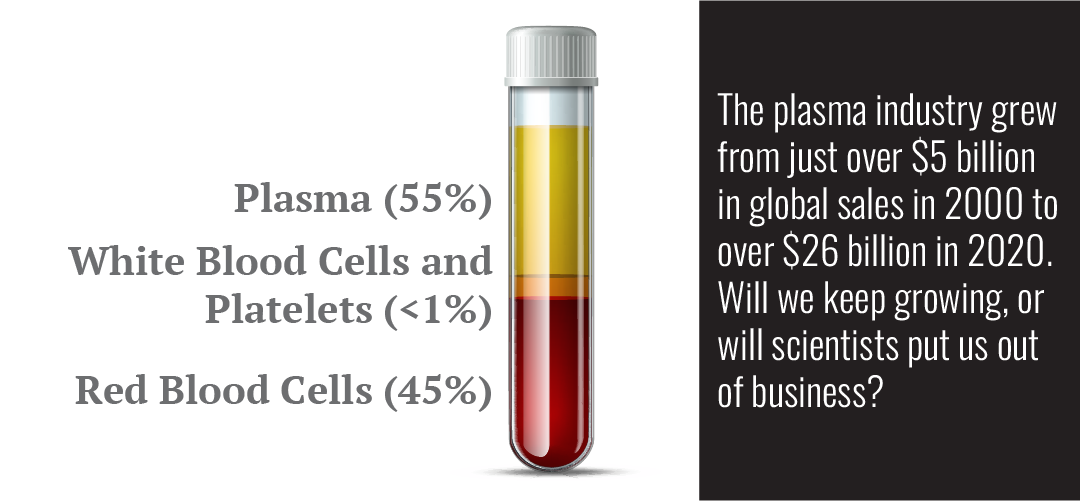
There is good and bad news for our future. As science gets closer and closer to creating artificial blood, things look bleak, but at the same time, there are more and more uses being discovered for human blood plasma.

1. Artificial blood got closer to reality with a $46 million grant from the US government in January 2023
A University of Maryland School of Medicine physician-scientist is heading up a consortium of researchers working to develop a shelf-stable product for trauma patients, aided by a $46 million, four-year grant from the Defense Advanced Research Projects Agency (DARPA).
The goal is to create a bio-synthetic whole-blood product that can be freeze-dried for easy portability, storage and reconstitution:
- The program is employing artificial intelligence, state-of-the-art experimental platforms and multiple complimentary animal models.
- The product will be tested in trauma victims with complex multiple injuries, including shock and traumatic brain injury.
- The head researcher, Dr. Doctor (not a typo!), will work with ErythroMer, the artificial oxygen carrier he pioneered in earlier studies, using pharmacology, computational modeling and machine learning to optimize the combined product, which will include synthetic platelets and freeze-dried plasma.
Whether the research team, which includes nine universities and seven biotech firms, will succeed, and if the product can be cost-effectively produced, scaled, packaged and controlled for quality remains to be seen.
Other new therapies may also threaten the plasma collection industry:
- Gene therapy is being used for hemophilia B, representing a cure with just one infusion.
- New recombinant and non-factor products have been introduced in the area of hemophilia therapy (recombinant factor VIII, VIIa, IX, XIII, von Willebrand, albumin, antithrombin-III, C1 esterase inhibitor and thrombin). Some of these provide higher trough levels in hemophilia patients, allowing them to infuse less frequently.
- Additional recombinant proteins are under development: recombinant fibrinogen, alpha-1 antitrypsin and others.
- Several biotechnology companies have developed new monoconal antibodies that mimic the immune modulatory mode of action of igG in the treatment of some diseases.
- The first competition for immunoglobulins was commercialized in late 2021. Efforts are underway to produce human albumin in rice plants, which may result in additional competition for plasma-derived albumin.
- Monoclonal antibodies (mAB) to prevent Hereditary Angioedema (HAE) now compete strongly with plasma-derived C1-INH. Novel mAB and small molecules are in development for the treatment of Alpha-1 Antitrypsin Deficiency.
2. But there are also new products being developed using human plasma proteins that have not been commercialized before
There are more than 2,000 proteins in plasma, but only 15-20 have been commercialized. Here are some recent efforts to bring others to market:
a. Plasminogen (which dissolves the fibrin of blood clots and acts as a proteolytic factor in a variety of other processes) has been approved and launched.
b. Companies are exploring reconstituted HDL from plasma.
c. Others seeking to commercialize novel proteins are exploring new business models.
It is unlikely that a new protein will supplant IgG as the plasma industry driver anytime soon, but additional proteins will no doubt contribute to the industry’s success and benefit new patients within a few years.
Back To Top
3. New formulations on the horizon
The industry is also investigating new formulations for plasma-based products, which may increase the need for human plasma:
- Subcutaneous (SCIG) preparations have gained market acceptance, first for low-dose applications, and more recently for additional diseases with higher dosages, such as CIDP.
- Reconstitution vial sizes have made it possible to administer coagulation factors to infants, avoiding the need for ports.
- New formulations are in development for an inhaled A1Pl, which may increase its usefulness and/or decrease dosing.
4. New indications for plasma-based products
Plasma-based products are under investigation for more diseases:
- IVIG and SCIG for Myasthenia Gravis, Neuropathic Pain, Secondary Immunodeficiencies, Dermatomyositis, etc.)
- Albumin in Cirrhosis, liver disease, Alzheimer’s Disease, Malaria and Sepsis
- Alpha-1 antitrypsin in lung Graft-vs-Host Disease and lung injuries
- Fibrinogen in aortic aneurysm surgery with acquired fibrinogen deficiency
- C1-0INH for antibody-mediated rejection in organ transplantation
Within the industry, there is much optimism for treatments not yet identified or developed.
Back To Top